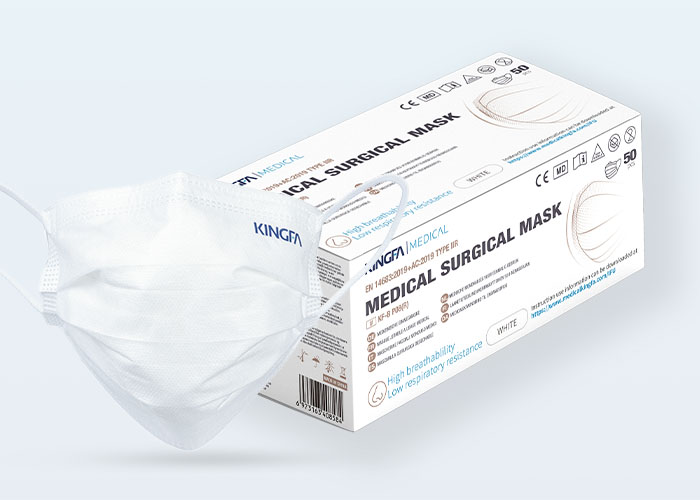
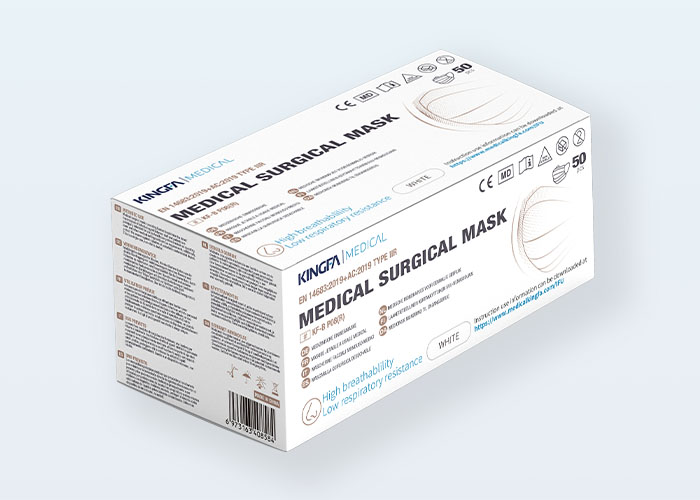
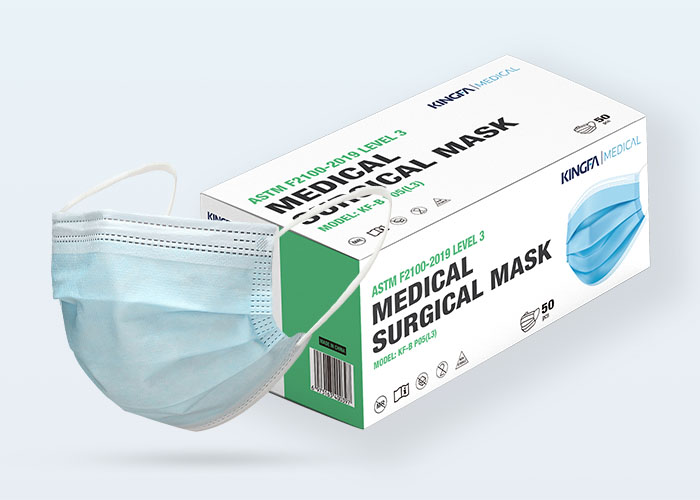
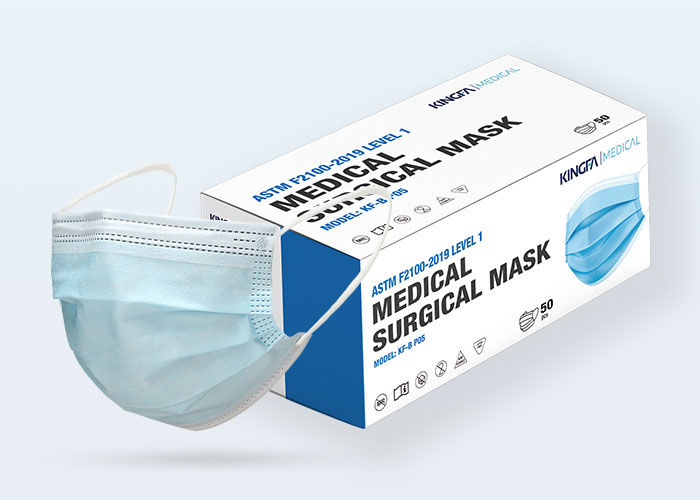
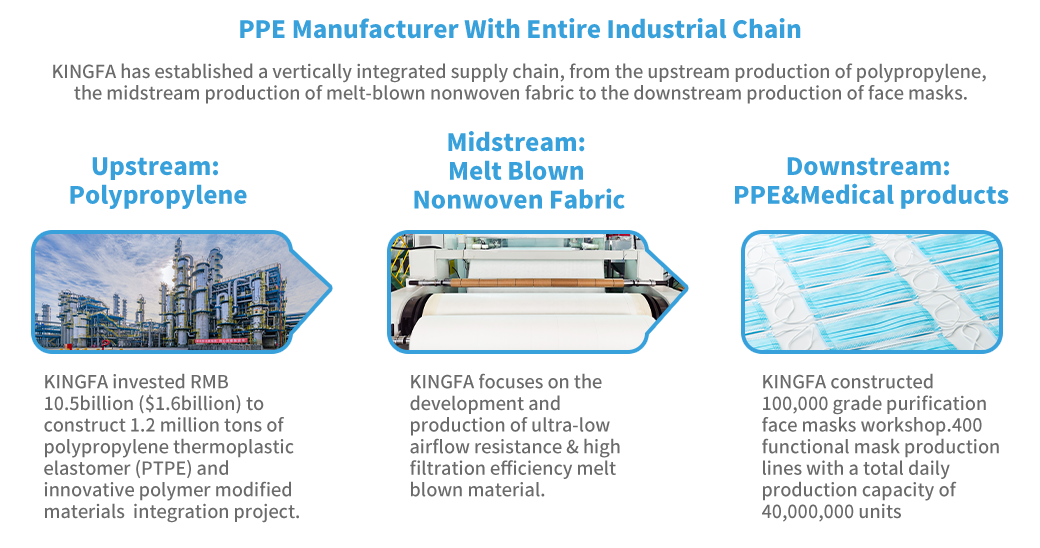
Type: | 3 Ply, Ear Loop, Hidden Bridge Crossbar |
Level: | Type IIR |
BFE: | ≥98% |
Material: | 2 Non-Woven Layers, 1 High Breathability Melt-Blown Layer |
Differential pressure: | < 20 Pa/cm² ,Triple Ventilation |
Packaging: | 50 Pcs, OEM Available |
Shelf-life: | 2 Years |
Application
KINGFA Type IIR Medical surgical masks providing barrier to minimize the direct transmission of infective agent between staff and patients are principally intended for use by healthcare professionals in an operating room and other medical settings with similar requirements.Under the same filtration efficiency, it has more than 50% lower ventilation resistance than ordinary corona electret products, making breathing more smoothly.
Breathable Technology
KINGFA has developed an organic-inorganic composite electret and use ultrapure high-speed water tribo-charging technology in manufacturing the melt-blown fabric. The long-lasting water tribo-charging technology has lower ventilation resistance. Under the same filtration efficiency, it has more than 50% lower ventilation resistance than ordinary corona electret products, making breathing more smoothly.
Fully Electret Technology (Melt Blown Fabric)
Using ultra-pure water friction charging principle and special charge capture technology, the charge of fiber is greatly increased, so as to significantly improve the filtration efficiency and reduce the respiratory resistance.
Low VOC and low odor melt-blown materials
KINGFA has independently designed a complete set of steam stripping/thermal desorption low odor equipment. The high melt-flow index melt-blown polypropylene material prepared via the steam extraction low-odor process has lower odor and less peroxide residue.
Features
●Made of 3 layer material. Filters at least 98% of particles.
●Differential pressure (Pa/cm²):< 20 Pa ,Triple ventilation
●Powered air-purifying respirators (PAPRs) may have much lower inhalation resistance but the same exhalation resistance.
●Premium materials ensure mask will not lint or tear.
●Specially designed inner facing improves comfort.
●Skin friendly, odorless, fiber-glass free and latex-free.
●Soft elastic ear loop design can help to fit different human faces
●The adjustable nose bridge clip provides a secure fit for better protection.
Quality Standards
●Standard: Type IIR, EN14683:2019+AC: 2019
●Product Classification: Class I Medical Device(Annex VIII of the MDR)
Material
Upgraded material for better protection. Type IIR Disposable Medical Masks are made of 3 layers with 1 non-woven outer layers as well as 1 non-woven inner layers and a high breathability melt-blown layer to absorb small moisture particles.
Technical Specifications
●Bacterial filtration efficiency (BFE): ≥ 98%
●Differential pressure (Pa/cm²): < 20 Pa
●Splash resistance (kPa): ≥ 16,0 Pa
●Microbiological Cleanliness(cfu/g):≤ 30
Logistic Information
●Packaging: 50pcs/box, 50boxes/carton
●Box Size:190*100*80mm,Carton Size:525*395*420mm
●Box Gross Weight:207±10g, Carton Gross weight:11520±500g
Container Loading
●20GP: 280 cartons (700,000 units)
●40GP: 600 cartons (1,500,000 units)
●40HQ: 750 cartons (1,875,000 units)
OEM Services
●Product design and development
●Flexible & timely manufacture
●Quality control and regulatory compliance
●Cost effective with product assurance
●Packaging & shipment
●Technical support
Manufacturing Accreditations
●ISO 9001 Quality Management System
●ISO 13485 Quality Management System
●QSR820 FDA Quality System Requirements

Application
KINGFA Type IIR Medical surgical masks providing barrier to minimize the direct transmission of infective agent between staff and patients are principally intended for use by healthcare professionals in an operating room and other medical settings with similar requirements.Under the same filtration efficiency, it has more than 50% lower ventilation resistance than ordinary corona electret products, making breathing more smoothly.
Breathable Technology
KINGFA has developed an organic-inorganic composite electret and use ultrapure high-speed water tribo-charging technology in manufacturing the melt-blown fabric. The long-lasting water tribo-charging technology has lower ventilation resistance. Under the same filtration efficiency, it has more than 50% lower ventilation resistance than ordinary corona electret products, making breathing more smoothly.
Fully Electret Technology (Melt Blown Fabric)
Using ultra-pure water friction charging principle and special charge capture technology, the charge of fiber is greatly increased, so as to significantly improve the filtration efficiency and reduce the respiratory resistance.
Low VOC and low odor melt-blown materials
KINGFA has independently designed a complete set of steam stripping/thermal desorption low odor equipment. The high melt-flow index melt-blown polypropylene material prepared via the steam extraction low-odor process has lower odor and less peroxide residue.
Features
●Made of 3 layer material. Filters at least 98% of particles.
●Differential pressure (Pa/cm²):< 20 Pa ,Triple ventilation
●Powered air-purifying respirators (PAPRs) may have much lower inhalation resistance but the same exhalation resistance.
●Premium materials ensure mask will not lint or tear.
●Specially designed inner facing improves comfort.
●Skin friendly, odorless, fiber-glass free and latex-free.
●Soft elastic ear loop design can help to fit different human faces
●The adjustable nose bridge clip provides a secure fit for better protection.
Quality Standards
●Standard: Type IIR, EN14683:2019+AC: 2019
●Product Classification: Class I Medical Device(Annex VIII of the MDR)
Material
Upgraded material for better protection. Type IIR Disposable Medical Masks are made of 3 layers with 1 non-woven outer layers as well as 1 non-woven inner layers and a high breathability melt-blown layer to absorb small moisture particles.
Technical Specifications
●Bacterial filtration efficiency (BFE): ≥ 98%
●Differential pressure (Pa/cm²): < 20 Pa
●Splash resistance (kPa): ≥ 16,0 Pa
●Microbiological Cleanliness(cfu/g):≤ 30
Logistic Information
●Packaging: 50pcs/box, 50boxes/carton
●Box Size:190*100*80mm,Carton Size:525*395*420mm
●Box Gross Weight:207±10g, Carton Gross weight:11520±500g
Container Loading
●20GP: 280 cartons (700,000 units)
●40GP: 600 cartons (1,500,000 units)
●40HQ: 750 cartons (1,875,000 units)
OEM Services
●Product design and development
●Flexible & timely manufacture
●Quality control and regulatory compliance
●Cost effective with product assurance
●Packaging & shipment
●Technical support
Manufacturing Accreditations
●ISO 9001 Quality Management System
●ISO 13485 Quality Management System
●QSR820 FDA Quality System Requirements