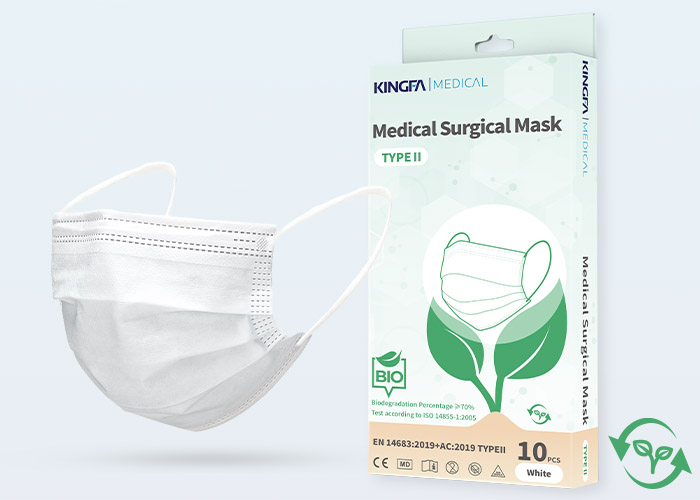
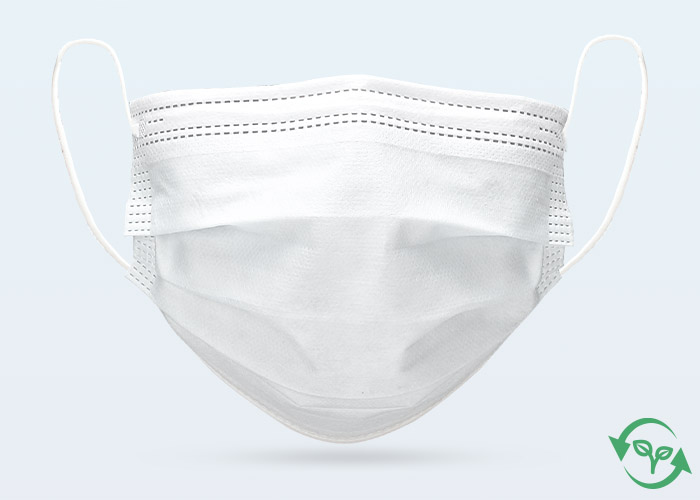
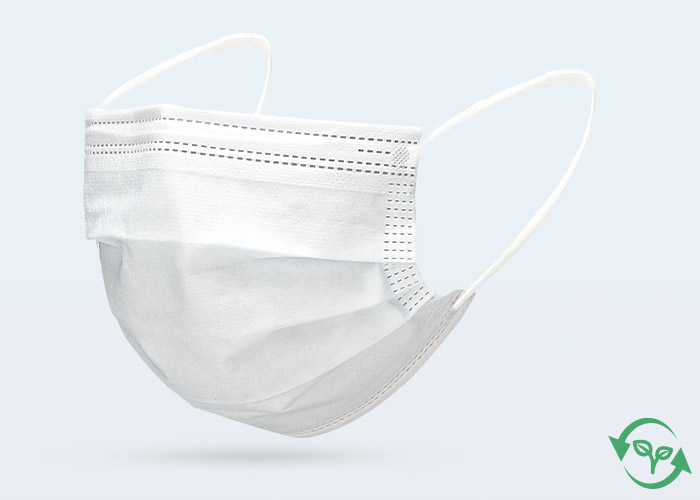
Type: | 3 Ply, Bio-Degradable, Ear-Loop, Hidden BridgeCrossbar |
Level: | Type II |
BFE: | ≥98% |
Material: | PLA Non-Woven Fabric,High Fluidity Melt-Blown PLA |
Packaging: | 10 Pcs, OEM Available |
Shelf-life: | 12 Months |
Why Choose KINGFA Biodegradable Medical Surgical Masks?
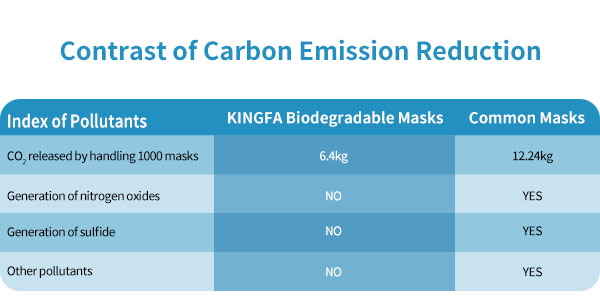
According to the survey of Environmental Science and Technology magazine in the United States, the number of masks used and discarded in the world each month has reached 129 billion, with a weight of about 640000 tons. Due to the use of polypropylene and other materials, it takes 450 years for an ordinary mask to be naturally degraded, and hundreds of years for it to be naturally degraded after centralized burial. If incineration is divided, waste gas will be discharged and pollute the air.KINGFA biodegradable mask adopts 100% biodegradable PLA, which greatly reduces the environmental pollution caused by the mask and the carbon emissions generated in production and recycling.
Application
KINGFA TYPE II Biodegradable Face Masks providing barrier to minimize the direct transmission of infective agent between staff and patients are principally intended for use by healthcare professionals in an operating room and other medical settings with similar requirements.
Protection & Breathability
According to the authoritative TUV test report, the bacterial filtering efficiency of KINGFA biodegradable face masks are above 99.8%, and respiratory resistance is no more than 35Pa, which meets the requirements of TYPE II of the European Union medical masks EN 14683 standard.
Features
●100% Fully Biodegradable PLA
●The MASK provides the same coverage, form, and function as traditional face masks, but in a biodegradable format.
●Made of 3 layer PLA material. Filters at least 98% of particles.
●The bio-degradation test of the nonwoven mask also includes the nose bridge and earloops
●Premium materials ensure mask will not lint or tear.
●Specially designed inner facing improves comfort.
●Skin friendly, odorless, fiber-glass free and latex-free.
●Soft elastic ear loop design can help to fit different human faces
●The adjustable nose bridge clip provides a secure fit for better protection.
●Thin & light multi-layer design reducing respiratory resistance.
Quality Standards
●Standard: Type II, EN14683:2019+AC: 2019
●Product Classification: Class I Medical Device(Annex VIII of the MDR)
Material
Upgraded material for better protection. KINGFA TYPE II Biodegradable Face Masks are made of 3 layers with 2 PLA non-woven layers and a high fluidity melt-blown PLA to absorb small moisture particles.
Biocompatibility
●In vitro cytotoxicity Test - Passed
●Delayed Skin Sensitization Test – Passed
●Primary Skin Irritation Test - Passed
Technical Specifications
●Bacterial filtration efficiency (BFE): ≥ 98%
●Differential pressure (Pa/cm²) : < 40
●Microbiological Cleanliness(cfu/g)≤2.4mbar
●Breathing resistance(Exhalation, 1600L/min) : ≤30
●Biodegradation Rate:76.71% (in absolute),77.10% (relative to reference material)
Logistic Information
●Packaging: 10pcs/box, 100boxes/carton
●Box Size:100*210*280mm,Carton Size:585*520*260mm
●Box Gross Weight:207±10g, Carton Gross weight:11520±500g
Container Loading
●20GP: 375 cartons (375,000 units)
●40GP: 750 cartons (750,000 units)
●40HQ: 850 cartons (850,000 units)
OEM Services
●Product design and development
●Flexible & timely manufacture
●Quality control and regulatory compliance
●Cost effective with product assurance
●Packaging & shipment
●Technical support
Manufacturing Accreditations
●ISO 9001 Quality Management System
●ISO 13485 Quality Management System
●QSR820 FDA Quality System Requirements

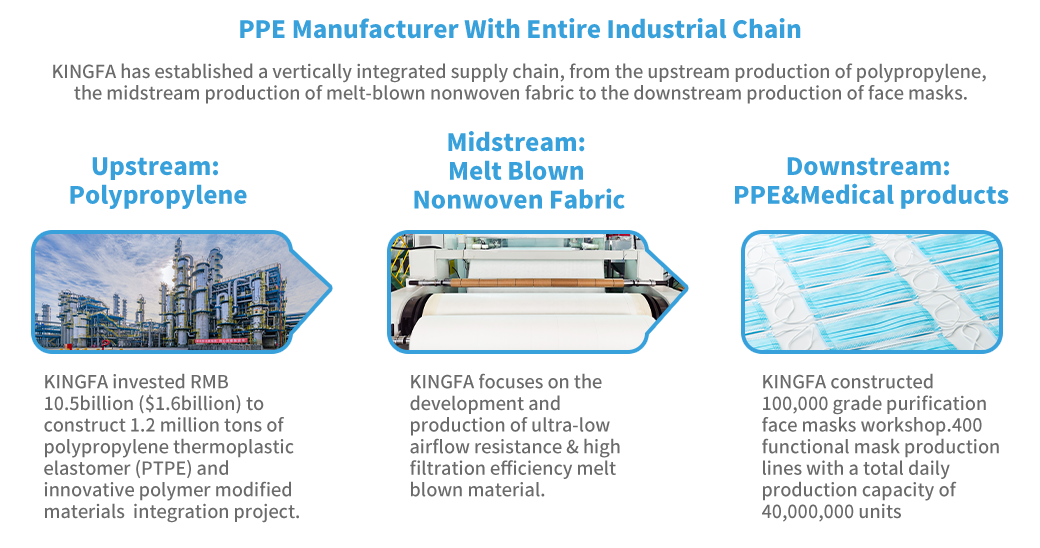
Why Choose KINGFA Biodegradable Medical Surgical Masks?
According to the survey of Environmental Science and Technology magazine in the United States, the number of masks used and discarded in the world each month has reached 129 billion, with a weight of about 640000 tons. Due to the use of polypropylene and other materials, it takes 450 years for an ordinary mask to be naturally degraded, and hundreds of years for it to be naturally degraded after centralized burial. If incineration is divided, waste gas will be discharged and pollute the air.KINGFA biodegradable mask adopts 100% biodegradable PLA, which greatly reduces the environmental pollution caused by the mask and the carbon emissions generated in production and recycling.

Application
KINGFA TYPE II Biodegradable Face Masks providing barrier to minimize the direct transmission of infective agent between staff and patients are principally intended for use by healthcare professionals in an operating room and other medical settings with similar requirements.
Protection & Breathability
According to the authoritative TUV test report, the bacterial filtering efficiency of KINGFA biodegradable face masks are above 99.8%, and respiratory resistance is no more than 35Pa, which meets the requirements of TYPE II of the European Union medical masks EN 14683 standard.
Features
●100% Fully Biodegradable PLA
●The MASK provides the same coverage, form, and function as traditional face masks, but in a biodegradable format.
●Made of 3 layer PLA material. Filters at least 98% of particles.
●The bio-degradation test of the nonwoven mask also includes the nose bridge and earloops
●Premium materials ensure mask will not lint or tear.
●Specially designed inner facing improves comfort.
●Skin friendly, odorless, fiber-glass free and latex-free.
●Soft elastic ear loop design can help to fit different human faces
●The adjustable nose bridge clip provides a secure fit for better protection.
●Thin & light multi-layer design reducing respiratory resistance.
Quality Standards
●Standard: Type II, EN14683:2019+AC: 2019
●Product Classification: Class I Medical Device(Annex VIII of the MDR)
Material
Upgraded material for better protection. KINGFA TYPE II Biodegradable Face Masks are made of 3 layers with 2 PLA non-woven layers and a high fluidity melt-blown PLA to absorb small moisture particles.
Biocompatibility
●In vitro cytotoxicity Test - Passed
●Delayed Skin Sensitization Test – Passed
●Primary Skin Irritation Test - Passed
Technical Specifications
●Bacterial filtration efficiency (BFE): ≥ 98%
●Differential pressure (Pa/cm²) : < 40
●Microbiological Cleanliness(cfu/g)≤2.4mbar
●Breathing resistance(Exhalation, 1600L/min) : ≤30
●Biodegradation Rate:76.71% (in absolute),77.10% (relative to reference material)
Logistic Information
●Packaging: 10pcs/box, 100boxes/carton
●Box Size:100*210*280mm,Carton Size:585*520*260mm
●Box Gross Weight:207±10g, Carton Gross weight:11520±500g
Container Loading
●20GP: 375 cartons (375,000 units)
●40GP: 750 cartons (750,000 units)
●40HQ: 850 cartons (850,000 units)
OEM Services
●Product design and development
●Flexible & timely manufacture
●Quality control and regulatory compliance
●Cost effective with product assurance
●Packaging & shipment
●Technical support
Manufacturing Accreditations
●ISO 9001 Quality Management System
●ISO 13485 Quality Management System
●QSR820 FDA Quality System Requirements