With the growing awareness of public health and increasing demand for industrial protection, nitrile gloves—an essential category of disposable protective gloves—are widely used across industries such as healthcare, laboratories, food processing, electronics manufacturing, and chemicals. To ensure product safety, efficacy, and market access, major global markets have established rigorous regulatory frameworks and technical standards, covering physical performance, biocompatibility, chemical resistance, and manufacturing quality systems. This article provides an in-depth analysis of regulatory requirements in the United States and the European Union, offering comprehensive compliance guidance for glove manufacturers, brand owners, and exporters.
1. United States Market: FDA Premarket Notification (510(k))
1.1 Legal Basis and Regulatory Authority
The U.S. Food and Drug Administration (FDA) regulates disposable medical gloves under Title 21 of the Code of Federal Regulations (21 CFR), through product registration, premarket notification, performance validation, and quality system control.
1.2 Product Classification and Applicable Regulations
Under FDA regulation, nitrile gloves are typically classified as:
• Examination Gloves (Class I medical devices): Partially exempt from GMP requirements, but most require submission of a 510(k) premarket notification.
• Surgical Gloves (Class II medical devices): Higher risk classification requiring full compliance with GMP and 510(k) clearance.
For gloves used for protective purposes in industrial or laboratory settings, FDA does not directly regulate them. However, they may need to comply with industry standards such as those set by OSHA or ASTM.
1.3 510(k) Premarket Notification
The 510(k) process is the core pathway to FDA compliance. Manufacturers must submit a technical dossier to demonstrate substantial equivalence to a legally marketed predicate device, including:
• Product description and intended use
• Performance testing (per ASTM D6319):
-Tensile strength, elongation at break
-Minimum thickness and weight
-Water leak test with an Acceptable Quality Level (AQL) of ≤2.5
• Biocompatibility testing (in accordance with ISO 10993):
-Skin irritation, sensitization, cytotoxicity
• Accelerated aging studies for shelf life verification
1.4 Quality System Requirements
Although Class I products may be partially exempt from GMP, most manufacturers are encouraged to comply with the FDA’s Quality System Regulation (QSR) under 21 CFR Part 820 to improve audit success and support global compliance.
2. EU Market: CE Marking and Applicable Regulations
2.1 Regulatory Framework
Nitrile gloves entering the EU market must follow the appropriate regulation based on intended use:
• Medical Gloves: Regulated under the Medical Device Regulation (MDR 2017/745), classified as Class I medical devices (non-sterile, non-measuring), requiring compliance with the EN 455 series.
• Personal Protective Gloves (PPE): Regulated under the Personal Protective Equipment Regulation (EU 2016/425), categorized by risk level from Category I to III. Chemical-resistant gloves fall under Category III and must comply with the EN 374 series.
2.2 Technical Documentation and Conformity Assessment
Different glove types follow different conformity procedures:
• MDR Class I:
-Self-declaration + technical documentation
• PPE Regulation Category III:
-Type examination (Module B) + production quality assurance (Module C2 or D)
-Requires assessment by a Notified Body and CE marking with Notified Body (NB) number
3. EN 455 Series: Core Standards for Medical Gloves
The EN 455 standard series is a mandatory requirement for nitrile medical gloves sold in the EU. It provides a full evaluation from pinhole detection to biocompatibility:
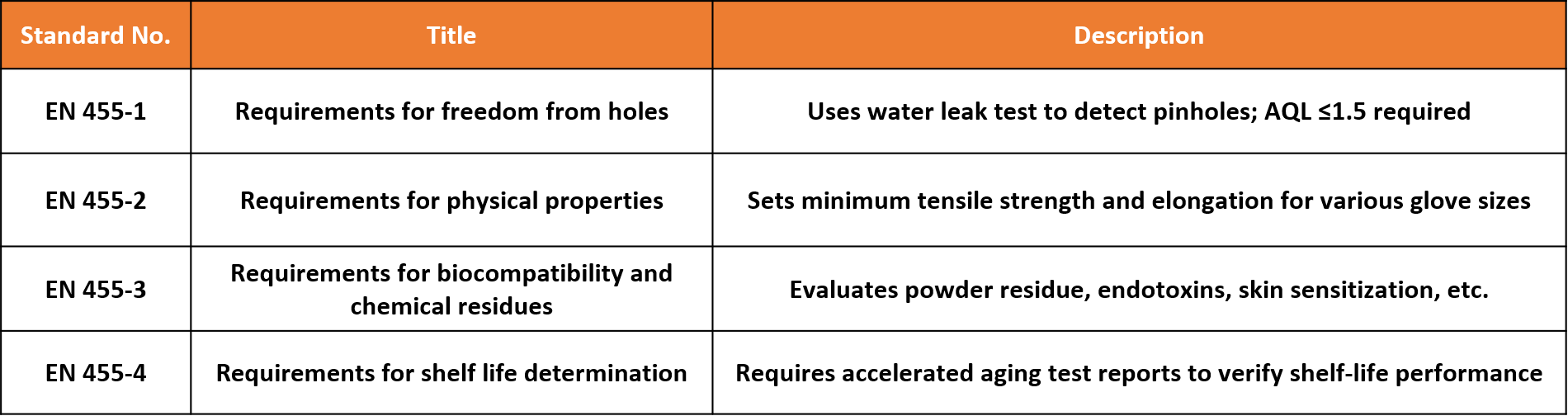
Only products compliant with the EN 455 series may be considered medical devices under MDR and bear the CE mark.
4. EN 374 Series: Standards for Chemical and Microbial Protection
For nitrile gloves intended for industrial, laboratory, or chemical handling applications, the EN 374 series provides the basis for evaluating chemical and biological protection:
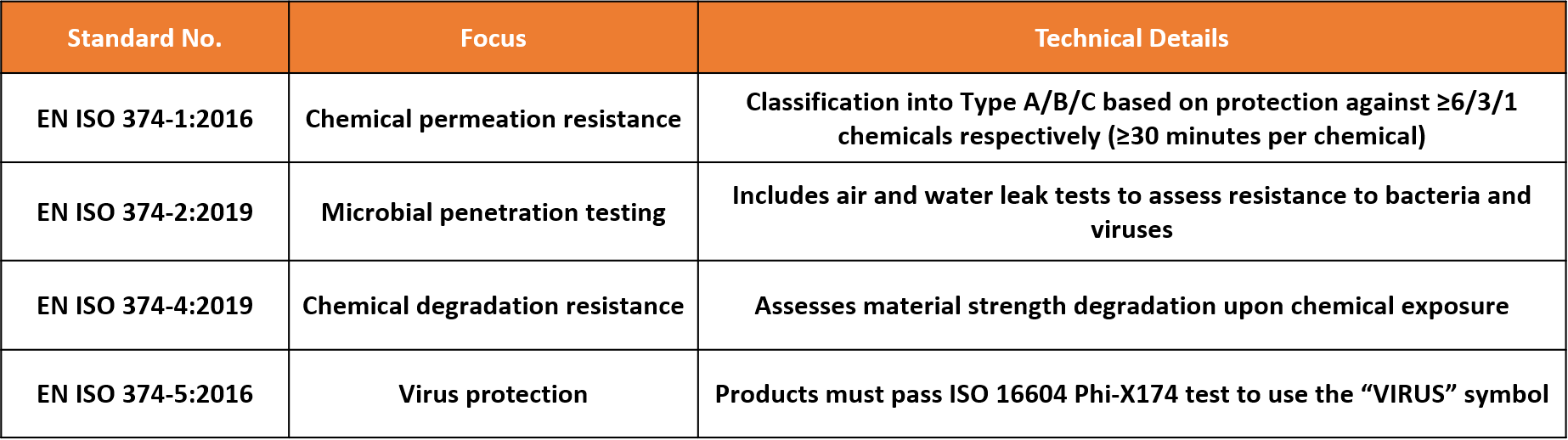
Marking Guidelines:
• Type A/B/C must be labeled with tested chemical codes (e.g., K: NaOH, P: H₂O₂).
• Gloves passing EN 374-5 viral testing may display the “VIRUS” pictogram.
5. Role of International Quality Management Systems
In addition to performance standards, major markets emphasize robust quality systems:
• ISO 13485: Required for medical devices, covering design, manufacturing, and post-market controls.
• ISO 9001: A general quality standard applicable to PPE.
• GMP Compliance: Required by the FDA and high-end markets such as Japan and Canada.
6. Comparison of Certification Systems by Market

7. Summary and Compliance Recommendations
In a globalized landscape, nitrile glove manufacturers aiming for multi-region, multi-purpose market coverage must strategically address different regulatory systems:
• Clarify Intended Use: Medical vs. industrial applications follow completely different regulatory routes. Dual compliance systems may be needed.
• Align R&D with Standards: Adopt both EN and ASTM standards at the product development stage to enhance global adaptability.
• Invest in Quality Systems: Certifications such as ISO 13485 and ISO 9001 not only improve product consistency but also facilitate regulatory audits.
• Collaborate with Compliance Bodies: Partner with reputable third-party testing and certification agencies (e.g., SGS, TÜV, Intertek) to enhance efficiency and precision.
Looking ahead, as sustainability becomes a regulatory priority, certification frameworks are expected to evolve toward biodegradability, safety, and carbon footprint traceability. Nitrile glove enterprises should take a proactive approach—enhancing not only product performance but also regulatory and brand competitiveness.




